Routine Activity
1- Diagnosis of Rabies in animals and Humans after and before death:
In most regions of the world, rabies in animals and humans is still diagnosed on the basis of clinical signs and symptoms. Rabies diagnosis can be performed on several different tissues or body secretion specimens, depending on the diagnostic techniques and the type of test that will be performed (ante-or post-mortem diagnosis). Post mortem diagnosis on brain tissue is the only validated procedure for laboratory diagnosis of rabies in animals.
This center receive brain, brain biopsy, skin biopsy and saliva samples from different regions of the country; these specimens are examined by the fluorescent antibody technique (FAT), the gold standard in rabies diagnosis, Mouse Inoculation Test (MIT) or Rapid Tissue Culture Inoculation Test (RTCIT) as confirmatory methods for post-mortem and molecular detection test (qRT-PCR), for ante-mortem diagnosis of rabies. For better detection, specific primers base on rabies strains circulating in Iran were designed and qRT-PCR re-launched.
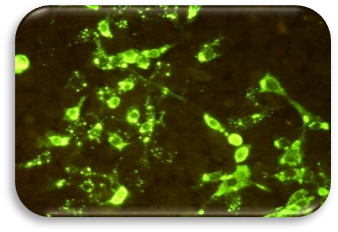
Some of the suspected specimens from north provinces of Iran, are examined in Amol rabies laboratory under the supervision of national Collaborating Center for Reference and Research on Rabies.
2- Serology Laboratory (Evaluation of anti-rabies Antibody, RFFIT):
In this laboratory, measuring of specific antibodies against rabies was carried by the rapid fluorescent focus inhibition test (RFFIT) on human or animal serum. Control of rabies immunoglobulin titers in imported anti-rabies serum as well as safety serum manufactured locally is performed using this method by staffs of the laboratory. In this laboratory, antibody titers were measured by ELISA faster than RFFIT that used in the screening.
In this laboratory, antiviral effects of some drugs is examined, as well as the virucidal effects of some imported or domestically disinfectants.
- Potency test for antirabies serum and immunoglobulin.
- Titration of Human Rabies Immunoglobulin which is imported and or produced by Blood Transfusion Organization of Iran by Rapid Fluorescent Focus Inhibition Test (RFFIT).
- Detection of immunity level against rabies in high risk vaccinated workers in different centers by Rapid Fluorescent Focus Inhibition Test (RFFIT) and ELISA.
- Different batches of human and animal rabies vaccine would be tested by NIH test.
- Human rabies immunoglobulin obtained from vaccinated persons and vaccinated pets would be titrated by RFFIT.

3- Cell Culture:
- Culture and maintaining the following cells: BHK, VERO, BSR and
- Neuroblastoma cells:
- Applying of the BHK and BSR cells for titration of rabies antibody by
- Rapid Fluorescent Focus Inhibition Test (RFFIT).
- Diagnosis of rabies by Neuroblastoma(N2a) cells.
- Production and purification of rabies virus in BHK and BSR cells.

4- Vaccine safety and tests for potency and antigen quantification:
- Human Rabies vaccine.
- Animal rabies vaccine.
Terms of Reference (TOR)
- TOR 1: To conduct and coordinate research on rabies, based on recommendations of the WHO expert committees and scientific groups’ recommendations.
- TOR 2: Upon WHO’s request, provide training in the fields of rabies epidemiology, laboratory diagnosis and PEP, based on the latest WHO's recommendations, as part of the global network of WHO collaborating centers on rabies.
- TOR 3: Upon WHO’s request, provide support & expertise for surveillance & control measures of rabies.
Activities and work plans for the next 4 years
- Post-Mortem Diagnosis of Rabies in suspected animals by Fluorescent Antibody Test (FAT) and Rabies Tissue Culture Infection Test (RTCIT)/Mouse Inoculation Test (MIT) along with setting up and optimization of new diagnostic methods.
- Assessment of Anti rabies antibody titer of Rabies Immunoglobulin in Human, Animal and product serum samples along with setting up and optimization of new methods.
- Cooperation in raising public awareness about rabies.
Research projects
- RNA detection of rabies virus in the brains of suspected rabid animals using RT-LAMP method compare to Real Time RT-PCR test as a quantitative molecular technique and DFA as a serologic method;
- Determination of the state of knowledge and attitudes of the general population of selected provinces in the field of rabies prevention in year 2019;
- Phylogeography, Population Dynamics, and Molecular Evolution of Iranian Bat Lyssaviruses;
- Adjuvanticity evaluation of cerium nanoparticles in veterinary rabies vaccine;
- Development and evaluation of Rabies Tissue Culture Infection Test (RTCIT) for diagnosis of rabies compare to Mouse Inoculation Test (MIT) in Fluorescent Antibody Test (FAT) -negative samples;
- Fractionation of the native Iranian snake venom (Naja naja oxiana) proteins by chromatography techniques (FPLC, HPLC) and study on the evaluation of their effects in rabies virus infectivity and detection of effective peptide in vitro;
- Study on the Molecular and Spatial Epidemiology of Rabies Virus in Iran's Border Provinces in 2017 in Comparison with Neighboring Countries in order to improve rabies surveillance system in Iran;
- Examination of rabies virus effect on apoptosis changes in mouse neuroblastoma cells (N2a) infected with a rabies virus in the substrate of neuroblastoma cell line;
- Study on virological ethiology of samples of encephalitis patiens admitted in medical university hospitals;
- Evaluation of Rabies Vaccination Efficacy through Rabies Antibody Titration Test for International Animal Movement by FAVN (Fluorescent Antibody Virus Neutralization) Method;
- Evaluation of map1lc3 gene expression in autophagy after inoculation of rabies in NMRI mouse model;
- The effects of Beclin-1 autophagy induction on the pathogenicity of rabies virus in the mouse model;
- Immuno-capture ELISA kit for measuring the glycoprotein content of rabies vaccine.
