Efficacy and Safety of Soberana/PastoCovac vaccine; A Randomized Clinical Trial in Pasteur Institute of Iran
A Phase III Study to evaluate the safety, and immunogenicity of a protein-based SARS-CoV-2 vaccine, Soberana/PastoCovac vaccine against COVID-19 has been published in the JAMA Network Open Journal (JAMA2023;6(5):e2310302).
Ehsan Mostafavi, director of the clinical trial project, provided insight into the vaccine's development process, stating that Cuba's Finlay Institute developed the Soberana2 and Soberana Plus vaccines, which were then produced at Pasteur Institute of Iran as PastoCovac and PastoCovac Plus after a successful technology transfer.
Publishing 16 papers related to the various stages of development and clinical studies of this vaccine
The completion of the joint Iran-Cuba Phase 3 clinical trial study of the Soberana/PastoCovac Covid-19 vaccine has yielded positive results, which have been published in the JAMA Network Open journal. JAMA medical journals are journals that have been published in the field of medicine since 1883 by the American Medical Association. JAMA Network Open is recognized as one of the most reliable clinical trial publishing journals globally.
Six papers featuring both Iranian and Cuban authors were among the 16 papers published on the development and clinical studies of Soberana (PastoCovac) vaccines.
Benefits of the PastoCovac vaccine
Soberana (PastoCovac) is a recombinant protein-based vaccine. It has emergency authorization for use in multiple countries and can serve as a booster dose for all vaccines in Iran, including for children aged 5-18. The vaccine has been approved for emergency use in Iran, Cuba, Belarus, Mexico, Nicaragua, and Venezuela. The vaccine offers affordable production, high efficacy, safety, and stability at 2-8 degrees Celsius. Its potential advantages, including fast and cost-effective production, make it a strong contender in the fight against the Covid-19 pandemic.
Vaccine results in terms of efficacy and safety in a recent study
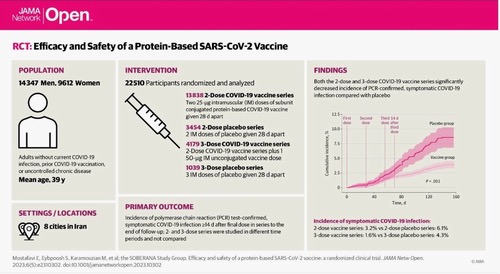
The clinical trial for the efficacy and safety of Soberana and Soberana Plus vaccines (known as PastoCovac and PastoCovac Plus in Iran) during phase three was conducted in eight cities across the country during the spring and summer of 2021.
The study included 23,959 people with an average age of 39 years. Approximately 29% of volunteers had at least one underlying disease. In July, 71% of the circulating variant was delta, and by August, it had increased to 95%.
In the two-dose regimen, 86% of people showed a four-fold increase in serum titer, while in the three-dose regimen, 99% of people experienced the same increase. Neutralizing antibodies were found in all participants who received three doses of the vaccine.
The vaccine was about 65% effective in preventing symptomatic forms of the disease in the three-dose regimen and about 97% effective in preventing hospitalization and severe forms of the disease.
No serious side effects were reported for any of the three injected doses of this vaccine. The most common side effect was pain at the injection site, observed in about 38% of volunteers after the first dose.
Vaccine Efficacy in Children
During this period, the clinical trial study of this vaccine was also conducted in children. The results which have been published showed that there was a four-fold increase in the antibody titer in 99% of children aged 3 to 11 years and 93% in children aged 12 to 18 years. Based on this, permission to inject the vaccine in children over 5 years of age was also issued for this vaccine in Iran.
The continuation of the effectiveness of this vaccine against the Omicron variant
Every laboratory study of the efficiency of vaccines against new variants needs to be approved at the community level for proper evaluation. Fortunately, in the two published studies of this vaccine, an acceptable neutralizing antibody titer was also shown against the Omicron variant. In addition, in a population-based study, the effectiveness with strong and durable protection of this vaccine has been demonstrated against symptomatic and severe diseases caused by Omicrons.
PastoCovac; Booster dose of the country's vaccines
Due to the emergence of new variants and the existence of evidence of a gradual decrease in the level of immunity resulting from the covid-19 vaccination, it is important to inject a booster dose after a few months, especially for vaccines based on the platform of the inactive virus. The results of studies at the Pasteur Institute of Iran showed that the PastoCovac vaccine can play an important role in the continuation of the vaccination program against this disease in the country.
According to the additional studies conducted at the Pasteur Institute of Iran, the PastoCovac vaccine was approved by the Food and Drug Organization as the first and second booster doses of all vaccines used in the country from the beginning of July 2022 and is used at vaccination centers across the country.
In the conducted studies it has been shown that the percentage of seroconversion (a 4-fold increase in antibody compared to the baseline value) of antibodies in the recipients of the PastoCovac booster dose was more than 3 times that of the Sinopharm vaccine group.
Also, the results of the immune stability evaluation in people who received 3 doses of the vaccines produced by the Pasteur Institute of Iran have shown an acceptable immune response in the recipients for at least six months.
These studies have shown that the efficacy of the covid-19 vaccines jointly produced by Iran's Pasteur Institute and Cuba's Finlay Institute as a booster dose in stimulating the immune system is significantly higher than that of the Sinopharm vaccine. In these studies, it has also been determined that the efficacy of the booster dose of PastoCovac and PastoCovac Plus vaccines was similar to each other and no specific side effects were reported after their injection.
Technology Transfer of the PastoCovac vaccine production
A few years ago, the Pasteur Institute of Iran entered into a contract with Cuba's Finlay Institute to transfer pneumococcal vaccine technology. However, with the onset of the COVID-19 pandemic, the Pasteur Institute of Iran realized that the technology used to make this vaccine was similar to that used in the development of the COVID-19 vaccines. As a result, the Institute amended the pneumococcal contract with the Cuban side and proceeded to develop a new vaccine. The researchers at the Pasteur Institute of Iran developed a production line using recombinant animal cells and acquired the necessary skills and technical knowledge to produce the vaccine.
To date, over 16 million doses of this vaccine have been produced and distributed, with the Pasteur Institute of Iran ready to produce more if required by the Ministry of Health. The Institute is proud to have provided an effective and safe vaccine to the people of Iran in collaboration with the Finlay Institute of Cuba. With over a hundred years of experience in producing various vaccines, the Pasteur Institute of Iran has instilled peace and security in the people, assuring them that they can safely use this vaccine and others produced by the Institute.